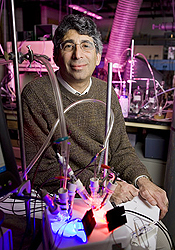
A novel experiment is taking place in the Princeton University lab of chemist Andrew Bocarsly. Like a battery, the experimental device has two poles of charged materials resting in a bath of chemical-laced water. A small tube bubbles carbon dioxide into the device, called a cell. The CO2 interacts with the charged metal coating one of the poles and, with the help of a special catalyst, begins to form bigger molecules that combine carbon, hydrogen, and oxygen atoms.
These bigger molecules have a more common name: hydrocarbons, the molecules that make up the fuels that power the modern world — coal, natural gas and oil. And what Bocarsly and his colleagues have done is essentially reverse combustion: they have taken the byproduct of burning fossil fuels — CO2, the greenhouse gas most responsible for climate change — and transformed it back into a fuel suitable for burning.
“The dominant thinking 10 years ago was that we should bury the CO2,” Bocarsly says. “If you could efficiently convert CO2 into something that was useful you wouldn’t have to spend all that money and energy to put it into the ground. You could sort of recycle it.”
The experiment in Bocarsly’s lab is part of an intensifying research effort to transform the copious energy from sunlight into liquid fuels by improving upon the work of plants, which, using only energy from the sun, take CO2, fuse it with hydrogen split from water, and make molecules to fuel growth. These ambitious energy projects would recycle CO2 emissions by allowing CO2 molecules to switch back and forth between byproducts of burning and building blocks of new fuel. It’s a potentially revolutionary technology, and the problem is not so much in pulling off the transformation — at least four different approaches to carry out “reverse combustion” either exist commercially or have been demonstrated in laboratories — but the high cost of doing so.
“Since the sun provides enough energy for our needs, our goal is to make a fuel using CO2 and sunlight — and maybe water — as feedstocks to produce the chemical fuel that can store the sun’s energy in a form that we can use where and when we need,” writes chemist Michael Berman of the U.S. Air Force Office of Scientific Research, which is funding much of the research. “We hope that this is something that can be done in an economically viable way.”
Attaining that goal remains a distant prospect. But the potential payoff of these long-shot experiments is potentially so great that the U.S. government, various labs, and some start-up companies are pumping sizeable amounts of money into the research. The technologies include producing methanol in a lab, harnessing microbes found in extreme environments to produce fuels, replicating the process of photosynthesis itself, and using sunlight to forge a synthetic fuel made of hydrogen and carbon monoxide.
Creating liquid light
In 2003, chemist Emily Barton took up a discarded experimental device that had been languishing in her mentor’s Princeton lab for more than a decade. She was searching for a novel solution to the growing problem of CO2 piling up in the atmosphere and changing the climate. The device — an electrochemical cell that transforms electricity into chemical reactions, or vice versa — employed an electrode made from the silvery white metal known as palladium and a catalyst called pyridinium, a byproduct of oil refining. When Barton’s predecessor and inventor of the device — Lin Chao — applied an electric current, the cell knitted CO2 into methanol, the simplest hydrocarbon.
When Chao had written about the device in 1994, it was largely ignored. But Barton reasoned that turning CO2 back into a useful product like methanol could provide a solution to the CO2 problem. Even better, she could tweak the device by adding a compound used for thin-film photovoltaic devices — gallium phosphide — and turn the cell into a solar-powered fuel maker.
Although this photovoltaic route is currently prohibitively expensive, venture capitalists have funded a start-up company — dubbed Liquid Light and based in New Jersey — to try to turn this electrochemical cell into the fuel refinery of the future. The company has replaced the expensive palladium electrode in the original with something cheaper and may not use pyridinium as the catalyst. “The only inputs we need are waste CO2, water and electricity,” says Liquid Light chairman and physicist Nety Krishna, noting the technology’s potential to simultaneously help solve two huge challenges — global warming and satisfying the world’s growing energy needs.
Tapping into ‘extremophiles’
From the various branches of the military to the Department of Energy, the U.S. government has a keen interest in alternatives to oil. In fact, the Advanced Research Projects Agency—Energy (ARPA-e) has created a program exclusively devoted to producing fuels from CO2.
“For every dollar the price of a barrel of oil goes up, the Navy spends $31 million more for fuel” per year, Secretary of the Navy Ray Mabus told an ARPA—e conference in March when announcing the military’s new collaborative effort with the fledgling energy agency. “Changing the way we produce and use energy is fundamentally about improving the national security of this country.”
Scientists are attempting to create electrofuels by using extremophiles — microbes that thrive in extreme environments.
The ARPA—e program for turning energy inputs into liquid fuel goes by the name of “electrofuels.” The scientists funded by the program attempt to create these electrofuels by harnessing the wonders of extremophiles — microbes that thrive in extreme environments, such as hydrothermal vents at the bottom of the ocean — to convert CO2 into fuels, using either electricity, hydrogen, or even ammonia.
That’s because extremophiles, unlike the vast majority of known life on the planet, make their living without photosynthesis. In fact, in the depths of the ocean, certain microbes rely on the energy in chemicals spewing from volcanic vents, while their extremophile peers more than mile underneath the planet’s surface rely on the slow decay of radioactive elements in the planet’s crust to thrive.
“We have bugs that go all the way,” making fuels from various energy inputs plus CO2, says chemist Eric Toone, deputy director for technology for ARPA—e and the electrofuels program manager. “I know it’s going to quote-unquote work. The interesting question now is: Is it going to matter?” The electrofuels program will only succeed in reducing the world’s oil addiction if it can produce fuels at a cost of roughly $60 per barrel — a price it is nowhere near achieving given the cost of electricity, hydrogen, and ammonia. “You’ve got to make this fuel at such a massive scale and such a low price,” Toone notes.
Another challenge is that the bugs themselves are not necessarily happy with the program. E. coli, Ralstonia eutropha, and the great microbial groupings of Pyrococcus and Rhodobacter, all want to use the extra energy to grow, not to make fuels. To force them to do so requires complex genetic and metabolic manipulation to ensure that as much energy as possible goes into fuel production.
Replicating photosynthesis
Researchers working outside the biological realm do not face the same constraints as a fuel-making microbiologist, or even a leaf. So the U.S. Department of Energy has hedged its ARPA—e bet by also investing in the Joint Center for Artificial Photosynthesis (JCAP) at the California Institute of Technology. The goal there is to build a system that works as well as photosynthesis in plants to produce fuel, whether hydrocarbons or just hydrogen.
“Chemical fuels would be the game-changer if you could directly make them efficiently and cost-effectively from sunlight,” says chemist Nathan Lewis, JCAP director.
Artificial systems can move energy as electrical current rather than the relatively chunky molecules that plants must rely upon in photosynthesis. In fact, an artificial system that uses photovoltaic panels to produce electricity, which is then used to split water into hydrogen and oxygen, can turn roughly 10 to 20 percent of incoming sunlight into the hydrogen gas that can fuel a hydrogen fuel cell. The most efficient photosynthetic plants — algae — only manage to turn roughly 3 to 6 percent of incoming sunlight into plant food.
So Lewis and his colleagues will have to build artificial light absorbers, molecule-makers, and even membranes to separate the various products of artificial photosynthesis, just as plants do. All of these components exist but do not necessarily work well together as a system. Within the next five years, JCAP hopes to prove that such a system can be created, and produce some fuel to prove it.
MIT’s Nocera predicts that enough energy to run a house could come from one drinking water bottle and sunlight.
Such a system has long been known by another name: the hydrogen economy. That hydrogen can be recombined with oxygen in a fuel cell to produce the electricity to drive an electric car or power a home. The problem with the hydrogen economy has always been the second word — the best hydrogen fuel cells rely on expensive platinum, and splitting water relies on expensive machinery. The most expensive cars on the planet are probably the hydrogen fuel cell test vehicles built by the likes of GM and Honda.
But a company called Sun Catalytix is attempting to make at least splitting water cheap, and thereby provide an inexpensive source of the hydrogen for fuel cells or to make hydrocarbons with CO2. Dropping the metal cobalt and the molecule phosphate into water as a catalyst and then running electricity through it — preferably supplied by the sun via a photovoltaic cell — can split water into hydrogen and oxygen. Chemist Dan Nocera of the Massachusetts Institute of Technology, whose team created the new catalyst — an invention somewhat erroneously hailed as an “artificial leaf” — predicts enough energy to run a house could be derived from one drinking water bottle in less than four hours of sunlight.
If hydrogen becomes cheap, then suddenly programs like electrofuels begin to make a lot more fiscal sense. “If you’ve got something you can drop in water and it evolves hydrogen, that’s pretty damn cool,” says Toone, which is why ARPA—e is also funding Sun Catalytix’s work. “We’ve seen the data and it actually works.”
Building a hydrocarbon
In the New Mexican desert, a six-meter wide dish of mirrors concentrates the sun’s rays on a half-meter-long cylindrical machine shaped like a beer keg. The mirrors focus sunlight through a window in the machine’s side, bathing a dozen, concentric rings in the sun’s heat. Temperatures quickly reach 1,500 degrees Celsius, which drives oxygen out of teeth made of iron oxide (rust) before the teeth rotate back into the dark side of the reactor. There the teeth suck oxygen back out of introduced steam or CO2, leaving behind hydrogen or carbon monoxide. When enough H2 and CO are produced, the mixture forms a very basic fuel known as synthesis gas, which is the building block used by the chemical industry to make hydrocarbons, chemicals, and even plastics.

Think of this keg-like machine as high-temperature, high-speed reverse rusting — and the expensive parts are not the inputs of CO2 or water, but rather the expense of the mirrors to harness the sun’s heat. “The real feedstocks are not CO2 and water, it’s sunlight,” says chemist James E. Miller of Sandia National Laboratory, co-inventor of the device. “Even though sunlight is free, what costs you most is collecting it and converting it into a useable form.”
Other groups are working on different designs or different materials, but the Sandia team in New Mexico estimates that it could make diesel or jet fuel for roughly $10 per gallon. There is another problem, however, one common to all such efforts to reverse combustion: To replace the more than 20 million barrels of oil consumed each day in the U.S. would require 62.4 trillion moles of pure CO2 per year. “If we go to a scale that is meaningful, where does the carbon come from?” Toone asks. “Learning how to recycle carbon is going to be important.”
Coal plants offer one source, producing roughly 500 pounds of CO2 per second when burning enough coal to generate one gigawatt of electricity, but that still isn’t enough to make a dent in transportation fuel use. Sucking CO2 out of the air remains prohibitively expensive, according to a recent report from the American Physical Society. But pulling CO2 out of seawater, where it is more highly concentrated, might offer one solution, as well as helping remedy the other peril from rising greenhouse gas concentrations in the atmosphere: ocean acidification.
Regardless, the first stirrings of a shift away from fossil fuels have started to show. The Princeton lab that gave birth to Liquid Light is now making butanol, the smallest molecule considered a hydrocarbon fuel, via the same process the lab used to make methanol. “We are making that unambiguously,” Bocarsly says. But “we’re in the early stages of understanding this.”